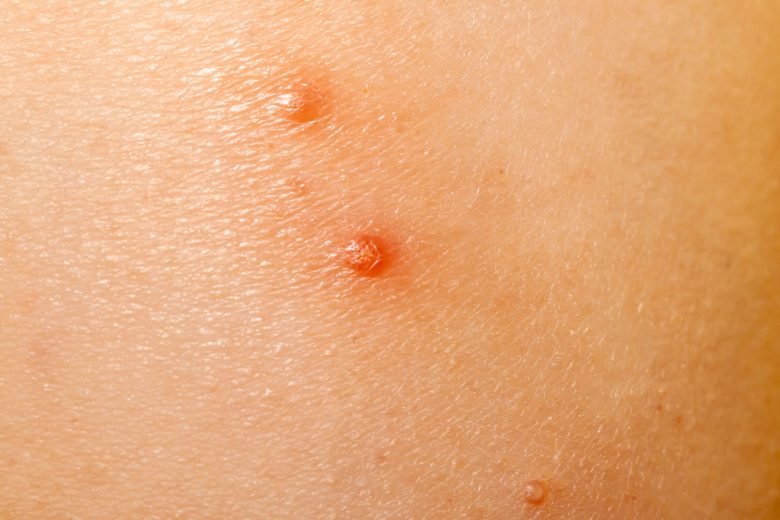
VP-102 could be the first approved drug on the market in the US to treat molluscum contagiosum.
Dermatology therapeutics company Verrica Pharmaceuticals Inc. (“Verrica”) announced the successful completion of the technology transfer of bulk solution manufacturing to Piramal Pharma Solutions.1 According to a company press release, the technology transfer includes the completion of the registration batch material, which has been placed on stability, and the manufacture of 3 process validation batches of bulk solution.
Verrica President and Chief Executive Officer Ted White said he believes the tech transfer will be well-received by the US Food and Drug Administration (FDA) and completes a substantial requirement toward the resubmission of their NDA for VP-102 for the treatment of molluscum in the first quarter of 2023.
VP-102 is a proprietary drug-device combination product that contains a GMP-controlled formulation of cantharidin (0.7& w/v) delivered via a single-use applicator that allows for precise topical dosing and targeted administration. Verrica has successfully completed a phase 2 study of VP-102 for the treatment of common warts and a phase 2 study for the treatment of external genital warts. Verrica intends to seek conditional approval to market VP-102 under the brand name YCANTH™.
If approved by the FDA, it will be the first product to treat molluscum contagiosum. The common, highly contagious skin disease affects an estimated 6 million people in the US. Most patients are children. Molluscum is caused by a pox virus that produces raised, pink-colored lesions that can cause pain, inflammation, itching and bacterial infections. Without treatment, it can last an average of 13 months to several years.
Reference
1. Verrica Pharmaceuticals Announces the Successful Technology Transfer of Bulk Solution Manufacturing of VP-102 to Piramal Pharma Solutions. https://wwwglobenewswirecom/news-release/2023/01/04/2582803/0/en/Verrica-Pharmaceuticals-Announces-the-Successful-Technology-Transfer-of-Bulk-Solution-Manufacturing-of-VP-102-to-Piramal-Pharma-Solutionshtml. January 4, 2023. https://verrica.com/. Accessed January 6, 2023.
The Weekly Roundup: January 2-6
The Cutaneous Connection: JAK Inhibitor Safety for Treating AD
I Used a New Dermal Filler: How Can This Get Me Arrested for Battery?
The Cutaneous Connection: Treating Atopic Dermatitis in the Winter
Opioid Use in Psoriatic Arthritis Is Associated With Higher Health Care Costs, Utilization
The Immune Profile of Merkel Cell Carcinoma
2 Clarke Drive
Cranbury, NJ 08512
609-716-7777
Author Profile
Latest entries
 राशीफल2024.04.16Aaj Ka Rashifal 16 अप्रैल 2024: आज इन 5 राशिवालों के लिए दिन लकी, निलेगा भाग्य का साथ – Hindustan
राशीफल2024.04.16Aaj Ka Rashifal 16 अप्रैल 2024: आज इन 5 राशिवालों के लिए दिन लकी, निलेगा भाग्य का साथ – Hindustan लाइफस्टाइल2024.04.16New Year Quotes: महान व्यक्तियों के 10 अनमोल विचार, अपना लिया तो नए साल से बदल जाएगी जिंदगी – अमर उजाला
लाइफस्टाइल2024.04.16New Year Quotes: महान व्यक्तियों के 10 अनमोल विचार, अपना लिया तो नए साल से बदल जाएगी जिंदगी – अमर उजाला विश्व2024.04.1611 अक्टूबर 2023 आज की ताजा खबरें और ब्रेकिंग समाचार हिंदी: हमास कमांडर को तबाह करके इजरायल कैसे ले रहा बदला? टीम इंडिया … – NBT नवभारत टाइम्स (Navbharat Times)
विश्व2024.04.1611 अक्टूबर 2023 आज की ताजा खबरें और ब्रेकिंग समाचार हिंदी: हमास कमांडर को तबाह करके इजरायल कैसे ले रहा बदला? टीम इंडिया … – NBT नवभारत टाइम्स (Navbharat Times) टेक2024.04.15Microsoft Build 2024 to unveil new AI features for Windows – The Indian Express
टेक2024.04.15Microsoft Build 2024 to unveil new AI features for Windows – The Indian Express